×
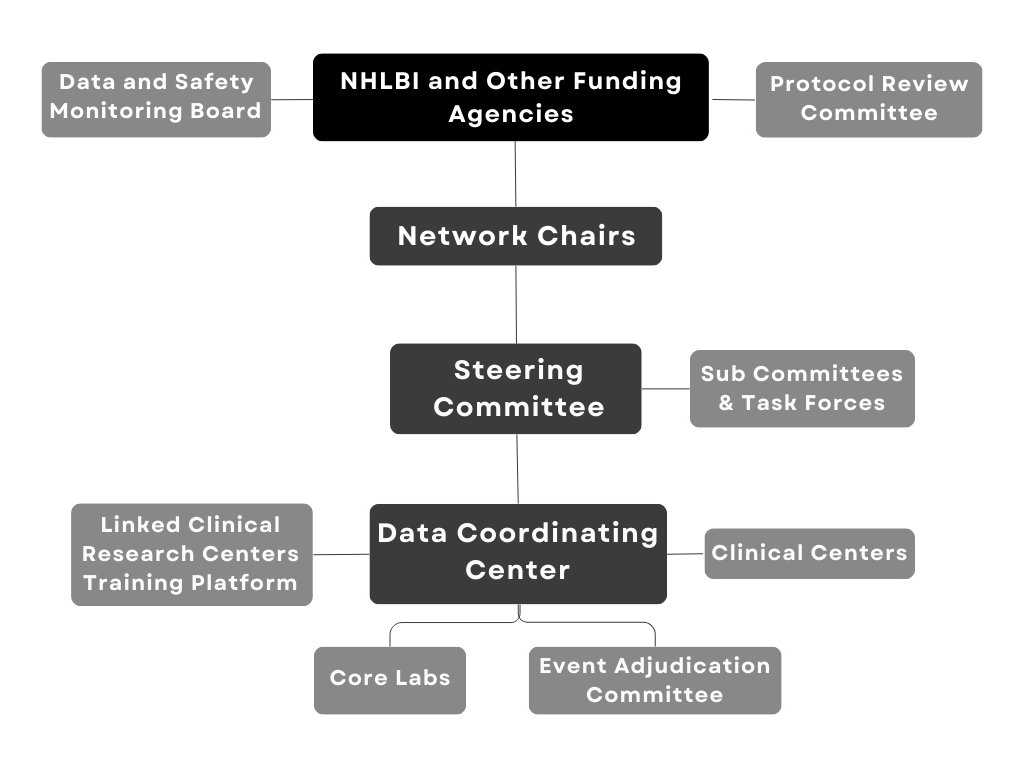
Data and Clinical Coordinating Center (DCC)
and International Clinical Coordinating Centers
The International Center for Health Outcomes and Innovation Research (InCHOIR) at the Icahn School of Medicine at Mount Sinai in New York City, functions as the Data and Clinical Coordinating Center for CTSN.
InCHOIR’s investigators and professional staff includes experts in clinical trial design and management, biostatistics, information technology, data management, regulatory affairs, safety and pharmacovigilance, administrative and financial management, and regulatory affairs.
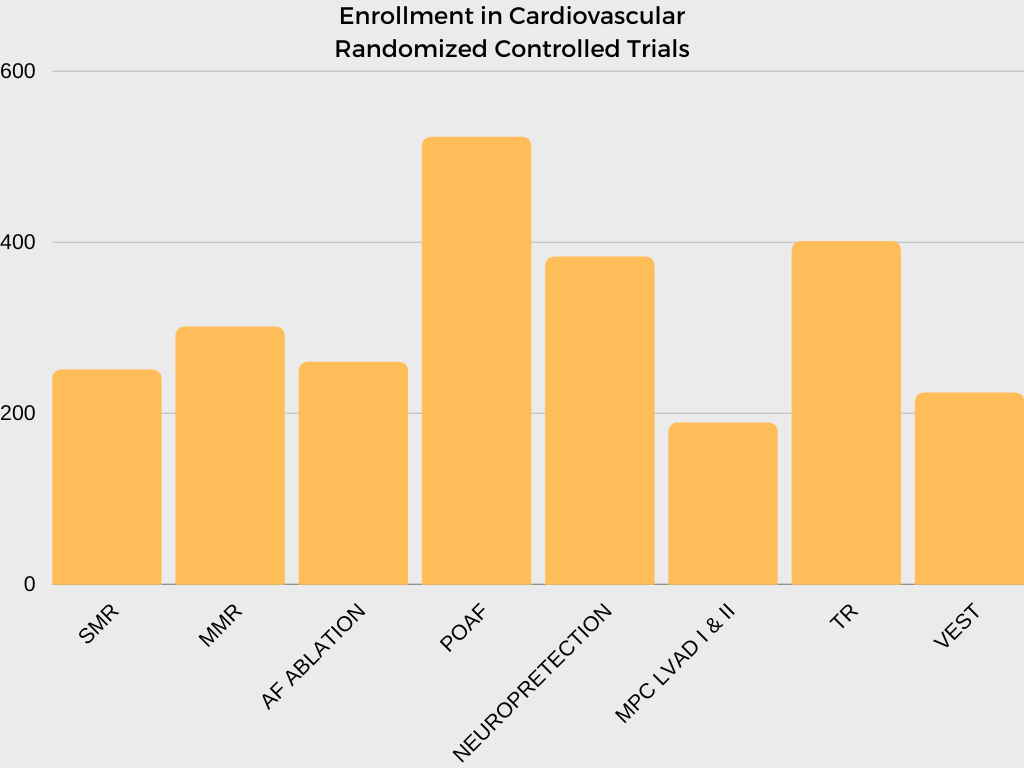
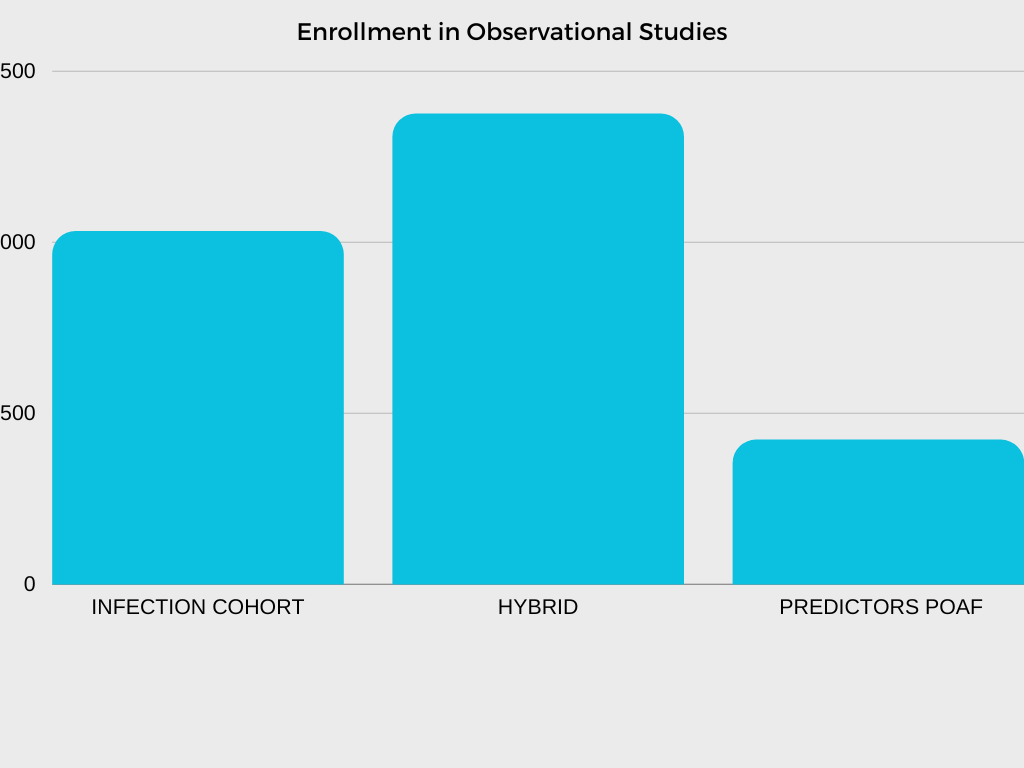
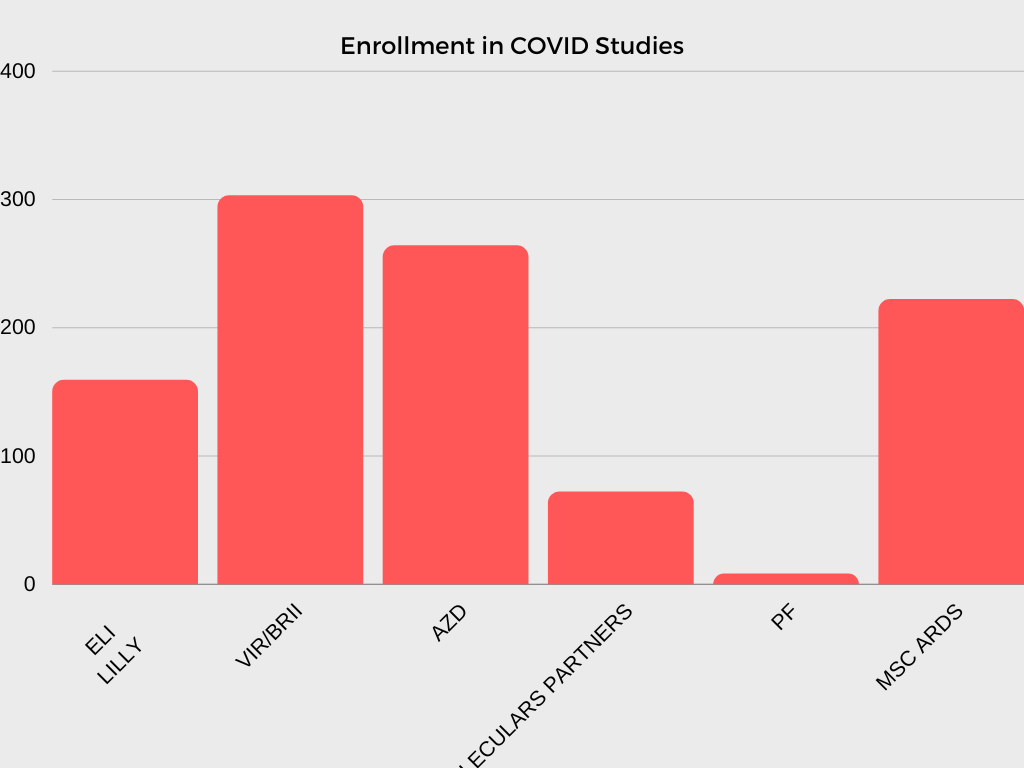
In collaboration with investigators and NHLBI program scientists, the CTSN DCC is responsible for developing multiple clinical trial protocols, establishing operational procedures and related materials for trial and study conduct, analyzing data, and interpreting and reporting results.